
Choose ANAVIP® to treat North American Pit Viper envenomation
IT’S ABOUT TIME
- Reconstitutes in seconds1
- Ready for dosing in minutes1
- Most envenomations were controlled within 4 hours2,a,b
- Low incidence of late venom effects up to 8 days1,b
- Circulates for weeks1
- Shelf-life of 4 years2
- Free replacements for future expiryc

Choose ANAVIP® to treat North American Pit Viper envenomation
IT’S ABOUT TIME
- Reconstitutes in seconds1
- Ready for dosing in minutes1
- Most envenomations were controlled within 4 hours2,a,b
- Low incidence of late venom effects up to 8 days1,b
- Circulates for weeks1
- Shelf-life of 4 years2
- Free replacements for future expiryc

Please choose your preferred platform:
Vimeo
YouTube
aMost patients achieved initial control in 1 or 2 infusions.
bThe efficacy analysis did not meet the prespecified statistically defined superiority criterion. However, the percentages of subjects showing prespecified criteria for coagulopathic effect on either Day 5 and/or Day 8 were 10.3% and 5.3% in the Groups 1 and 2 when compared to 29.7% in Group 3 indicating efficacy of ANAVIP in management of coagulopathic effect in patients with North American Pit Viper envenomation.
cReplacement policy available in accordance with state laws.
Please choose your preferred player:
Vimeo
YouTube
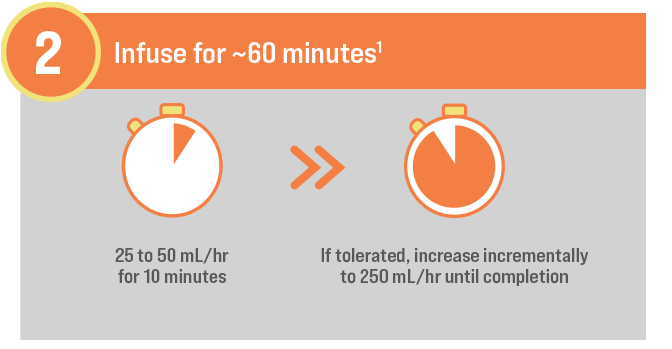
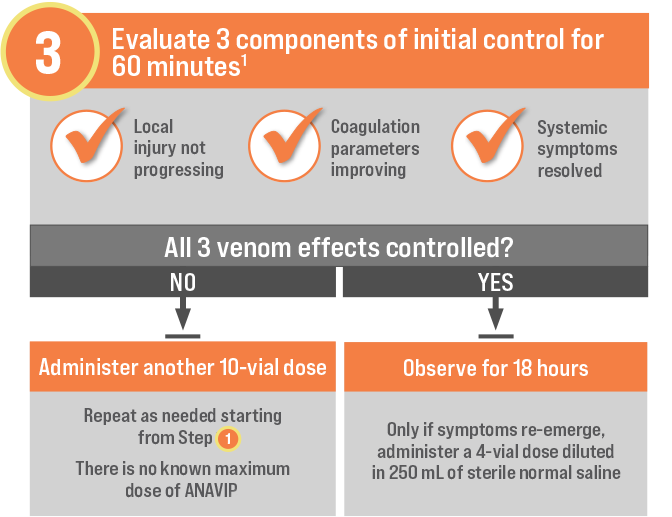
GO FROM SHELF TO SOLUTION IN MINUTES1
Before dosing, please read Dosage and Administration in the complete Prescribing Information. For Intravenous Use Only.
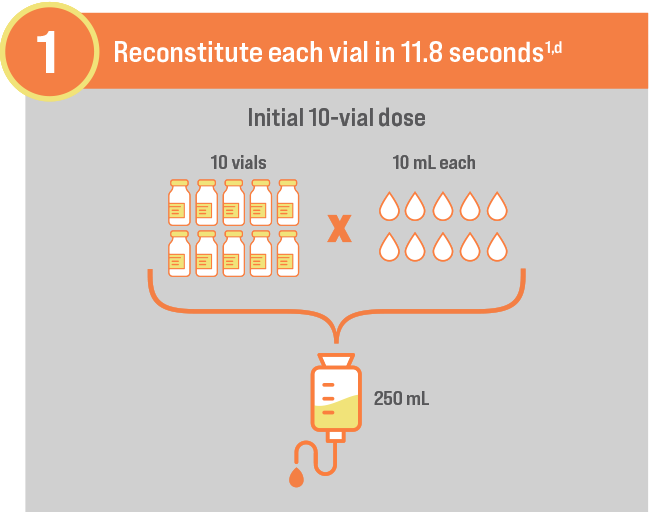
Store at room temperature1,e
Use within 6 hours of reconstitution1

dRange of 8 to 26 seconds per vial when using continuous gentle swirling with sterile normal saline.
eUp to 25°C (77°F). Brief temperature excursions are permitted up to 40°C (104°F).
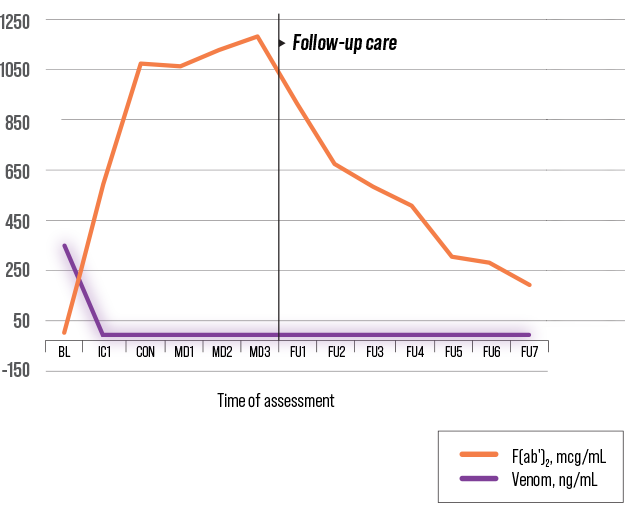
ANAVIP CONTROLLED LATE VENOM EFFECTS FOR UP TO 8 DAYS1,3,b
Study design: In a randomized, prospective, blinded, controlled, multicenter study of 121 patients experiencing envenomation in the United States, two ANAVIP regimens were compared with the approved regimen for the Fab Comparator. After achieving initial control, maintenance dosing was administered every 6 hours with either blinded study drug (Group 1 and Group 3) or placebo (Group 2). The efficacy endpoint was the proportion of patients experiencing a coagulopathic effect (defined as absolute platelet levels <150,000/mm3, absolute fibrinogen levels <150mg/dL, or clinical coagulopathy requiring intervention) on Day 5 or Day 8.1,3
bThe efficacy analysis did not meet the prespecified statistically defined superiority criterion. However, the percentages of subjects showing prespecified criteria for coagulopathic effect on either Day 5 and/or Day 8 were 10.3% and 5.3% in the Groups 1 and 2 when compared to 29.7% in Group 3 indicating efficacy of ANAVIP in management of coagulopathic effect in patients with North American Pit Viper envenomation.
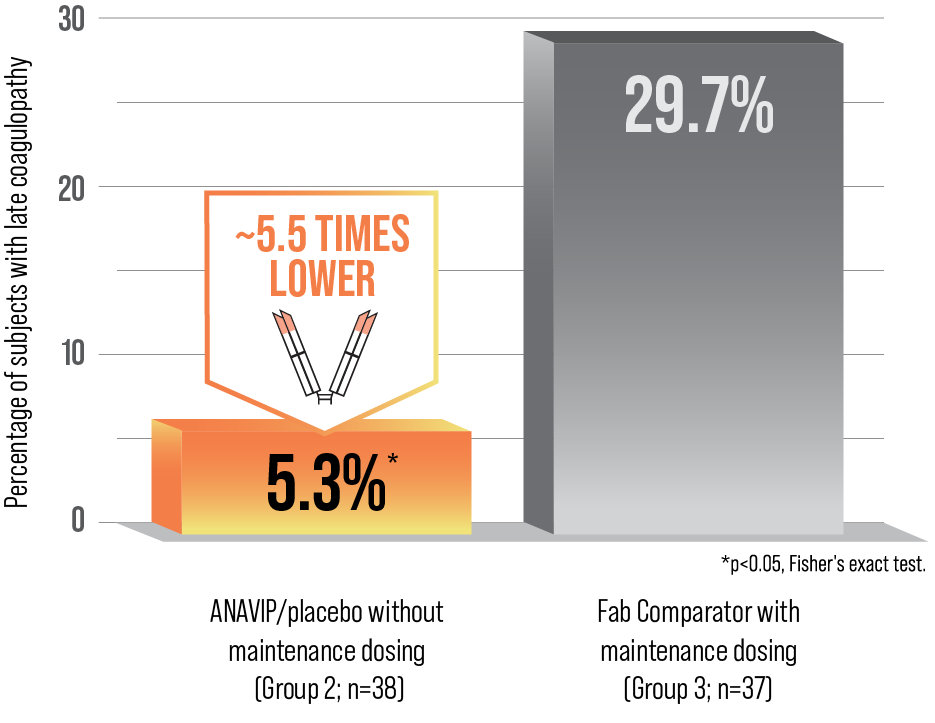
bThe efficacy analysis did not meet the prespecified statistically defined superiority criterion. However, the percentages of subjects showing prespecified criteria for coagulopathic effect on either Day 5 and/or Day 8 were 10.3% and 5.3% in the Groups 1 and 2 when compared to 29.7% in Group 3 indicating efficacy of ANAVIP in management of coagulopathic effect in patients with North American Pit Viper envenomation.
ANAVIP is indicated for all North American Pit Viper envenomations1

Cottonmouth

Copperhead

Rattlesnake
- Phase 3 study enrolled 99 Rattlesnake or Unknown Pit Viper bites, 21 Copperhead bites, and 1 Cottonmouth bite2,3
- ANAVIP controlled local, systemic, and hematologic symptoms for all patients studied2
- Zero patients in clinical trials required retreatment with ANAVIP for late venom effects (n=83)2-4
Swelling at the bite site was the only potential serious adverse event2,f
- While 9 patients experienced serious adverse events after receiving ANAVIP, all were considered unrelated to ANAVIP3,f
- The most common adverse reactions observed in more than 2 percent (2%) of patients in the clinical trials for ANAVIP were: pruritus, nausea, rash, arthralgia, peripheral edema, erythema, headache, myalgia, pain in extremity, and vomiting1
- 1 patient in each study group experienced acute serum reaction. All patients tolerated subsequent doses after resolution3
- 1 patient in each study group experienced symptoms suggestive of serum sickness. No immune reaction was severe3
Please see full Important Safety Information below and accompanying complete Prescribing Information for ANAVIP.
fOnly 1 serious adverse reaction (swelling in the bitten limb) was considered to be possibly related to ANAVIP, although the investigator subsequently wrote a note to file that attributed the symptoms to the North American Pit Viper envenomation.2
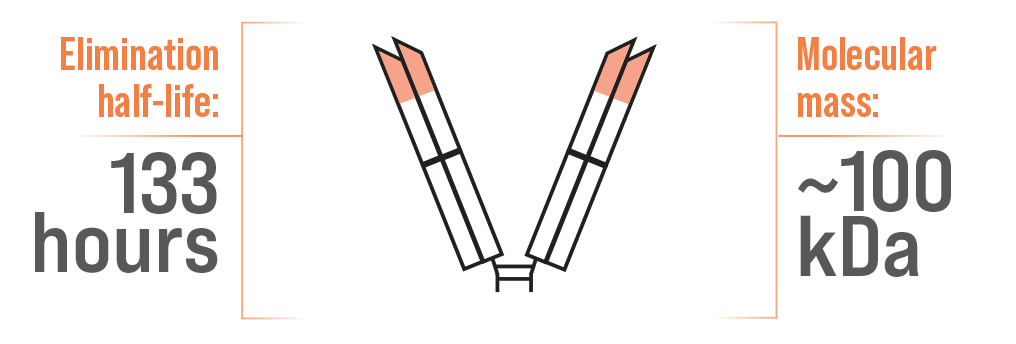
DESIGNED TO CIRCULATE FOR WEEKS1
F(ab′)2 – ANAVIP1,5

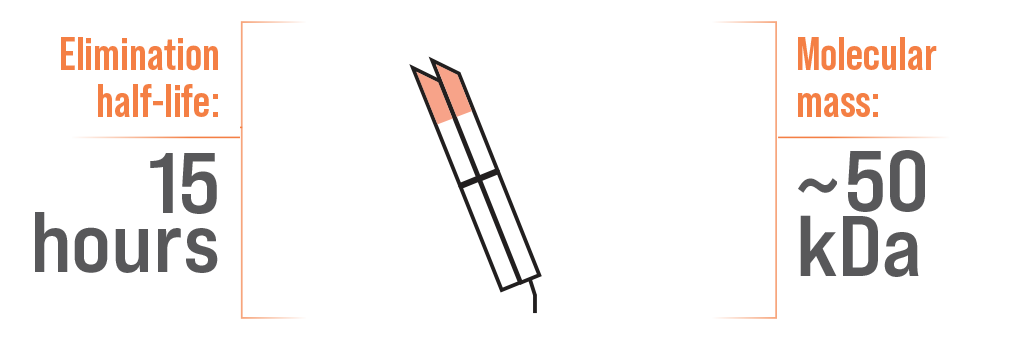
Single Fab Antivenom5,6

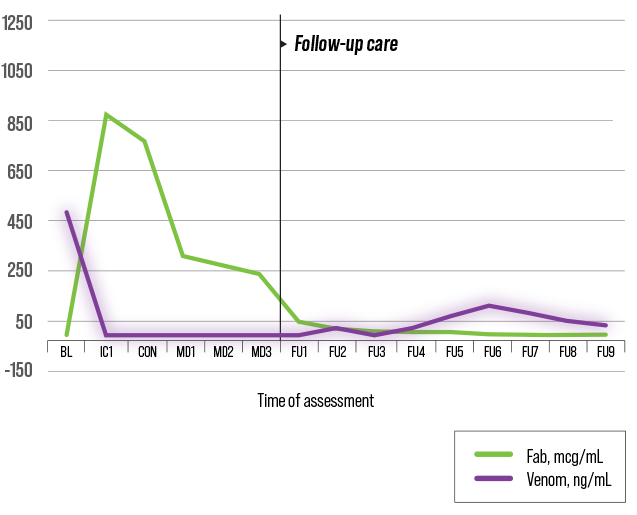
Study design: Randomized, open-label study in 12 adult subjects aged 18 to 70 to assess quantum serum venom levels.
PK, pharmacokinetic.
A difference in half-life has been attributed to molecular mass5,7
ANAVIP IS STABLE AT ROOM TEMPERATURE FOR UP TO 4 YEARS2
![ANAVIP® [crotalidae immune F(ab′)2 (equine)] carton and vial ANAVIP® [crotalidae immune F(ab′)2 (equine)] carton and vial](https://www.anavip-us.com/wp-content/uploads/2024/10/Anavip_Packaging_BoxVial_ANV-ca-008_small_trans6-e1727882802920.png)
Each carton NDC 66621-0790-2 contains 1 vial of ANAVIP NDC 66621-0790-1
- Free replacement for unused, expired ANAVIP (contact your wholesaler for details)
- Replacement vials are available 6 months prior to and 12 months after expirationg
- Initial doses are given in 10-vial increments, so make sure to order in 10-vial batches (see dosing information)
- Store at room temperature up to 25°C (77°F)
- Brief temperature excursions are permitted up to 40°C (104°F)
- DO NOT FREEZE
gExcept as otherwise required by applicable state law.
References:
- ANAVIP [crotalidae immune F(ab′)2 (equine)] Prescribing Information. Rare Disease Therapeutics, Inc.; Franklin, TN. June 2021.
- Data on file, Rare Disease Therapeutics, Inc.
- Bush SP, Ruha A-M, Seifert SA, et al. Comparison of F(ab′)2 versus Fab antivenom for pit viper envenomation: a prospective, blinded, multicenter, randomized clinical trial. Clin Tox. 2015;53:37-45.
- Boyer LV, Chase PB, Degan JA, et al. Subacute coagulopathy in a randomized, comparative trial of Fab and F(ab′)2 antivenoms. Toxicon. 2013;74:101-108.
- World Health Organization. (2018). WHO Expert Committee on Biological Standardization: WHO TRS No 1004. ANNEX 5: Guidelines for the production, control and regulation of snake antivenom immunoglobulins; 2017. https://www.who.int/bloodproducts/AntivenomGLrevWHO_TRS_1004_web_Annex_5.pdf Accessed March 26, 2021.
- CroFab [crotalidae polyvalent immune fab (ovine)] Prescribing Information. BTG International, Inc.; West Conshohocken, PA. August 2018.
- Gutiérrez JM, Leon G, Lomonte B. Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin Pharmacokinet. 2003;42:721-741.
IMPORTANT SAFETY INFORMATION
INDICATION
ANAVIP® [crotalidae immune F(ab′)2 (equine)] is an equine-derived antivenin indicated for the management of adult and pediatric patients with North American Pit Viper envenomation.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
Hypersensitivity
ANAVIP may cause allergic reactions.
- Patients with known allergies to horse protein are particularly at risk for an anaphylactic reaction. If signs or symptoms of anaphylaxis or hypersensitivity reactions (including urticaria, rash, tightness of the chest, wheezing, hypotension) occur, discontinue immediately and institute appropriate treatment.
- Monitor patients with follow-up visits for signs and symptoms of delayed allergic reactions or serum sickness (rash, fever, myalgia, arthralgia, pruritus, urticarial rash) and treat appropriately if necessary.
Transmissible Infectious Agents
ANAVIP is made from equine (horse) plasma and may therefore carry a risk of transmitting infectious agents, e.g., viruses.
Reactions to Cresol
Trace amounts of cresol from the manufacturing process are contained in ANAVIP. Localized reactions and generalized myalgias have been reported with the use of cresol as an injectable excipient.
ADVERSE REACTIONS
The most common adverse reactions observed in more than 2 percent (2%) of patients in the clinical trials for ANAVIP were: pruritus, nausea, rash, arthralgia, peripheral edemahttps://www.anavip-us.com/wp-admin/post.php?post=516&action=edit#, erythema, headache, myalgia, pain in extremity, and vomiting.
USE IN SPECIFIC POPULATIONS
- Pediatric Use: Twenty-four percent (21/86) of patients studied in clinical trials were 16 years of age or younger (6 patients were 2 years of age to 5 years of age, 15 patients ranged from at least 5 years of age to 16 years of age). None of the pediatric patients in the phase 3 study experienced a recurrent coagulopathic effect. All adverse reactions in the pediatric patients were non-serious. The most frequent adverse reactions among pediatric patients were nausea and vomiting, itching, and fever. Thus, the safety and efficacy in the pediatric population was not different from the adults.
Please see complete Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Rare Disease Therapeutics, Inc., at 1-844-472-7389 or by email at safety@raretx.com , or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
ANV-ISIF-001
IMPORTANT SAFETY INFORMATION
INDICATION
ANAVIP® [crotalidae immune F(ab′)2 (equine)] is an equine-derived antivenin indicated for the management of adult and pediatric patients with North American Pit Viper envenomation.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
Hypersensitivity
ANAVIP may cause allergic reactions.
- Patients with known allergies to horse protein are particularly at risk for an anaphylactic reaction. If signs or symptoms of anaphylaxis or hypersensitivity reactions (including urticaria, rash, tightness of the chest, wheezing, hypotension) occur, discontinue immediately and institute appropriate treatment.
- Monitor patients with follow-up visits for signs and symptoms of delayed allergic reactions or serum sickness (rash, fever, myalgia, arthralgia, pruritus, urticarial rash) and treat appropriately if necessary.
Transmissible Infectious Agents
ANAVIP is made from equine (horse) plasma and may therefore carry a risk of transmitting infectious agents, e.g., viruses.
Reactions to Cresol
Trace amounts of cresol from the manufacturing process are contained in ANAVIP. Localized reactions and generalized myalgias have been reported with the use of cresol as an injectable excipient.
ADVERSE REACTIONS
The most common adverse reactions observed in more than 2 percent (2%) of patients in the clinical trials for ANAVIP were: pruritus, nausea, rash, arthralgia, peripheral edema, erythema, headache, myalgia, pain in extremity, and vomiting.
USE IN SPECIFIC POPULATIONS
- Pediatric Use: Twenty-four percent (21/86) of patients studied in clinical trials were 16 years of age or younger (6 patients were 2 years of age to 5 years of age, 15 patients ranged from at least 5 years of age to 16 years of age). None of the pediatric patients in the phase 3 study experienced a recurrent coagulopathic effect. All adverse reactions in the pediatric patients were non-serious. The most frequent adverse reactions among pediatric patients were nausea and vomiting, itching, and fever. Thus, the safety and efficacy in the pediatric population was not different from the adults.
Please see complete Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Rare Disease Therapeutics, Inc., at 1-844-472-7389 or by email at safety@raretx.com, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
ANV-ISIF-001
